Select the correct option based on the statements below:
| Assertion (A): | The bond angle in ethers is slightly less than the angle of a tetrahedron. |
| Reason (R): | There is repulsion between the two bulky (— R) groups. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
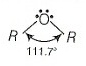
Hence, Assertion is false but Reason is true.

© 2026 GoodEd Technologies Pvt. Ltd.