Select the correct option based on the statements given below:
| Assertion (A): | The addition reaction of water to but-1-ene in an acidic medium yields butan-1-ol. |
| Reason (R): | Addition of water in an acidic medium proceeds through the formation of a primary carbocation. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
The addition reaction of water to but-1-ene in acidic medium yields butan-2-ol not butan-1-ol. When the addition of water in an acidic medium then the formation of secondary carbonation takes place not primary carbocation.
The secondary carbocation is more stable than the primary carbocation.
The mechanism is as follows:
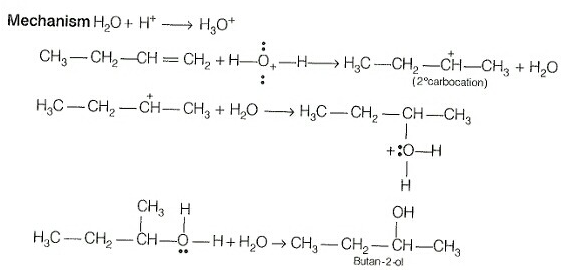
Hence, both Assertion and Reason are false.

© 2026 GoodEd Technologies Pvt. Ltd.