10.22 What happens when
(i) n-butyl chloride is treated with alcoholic KOH,
(ii) bromobenzene is treated with Mg in the presence of dry ether,
(iii) chlorobenzene is subjected to hydrolysis,
(iv) ethyl chloride is treated with aqueous KOH,
(v) methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN?
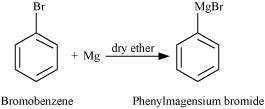
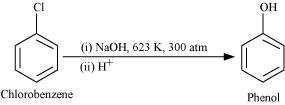

© 2026 GoodEd Technologies Pvt. Ltd.