10.17 Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH. NEETprep Audio Note (English):
r 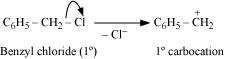
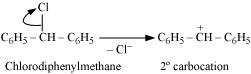
Hydrolysis by aqueous KOH proceeds through the formation of carbocation. If carbocation
is stable, then the compound is easily hydrolyzed by aqueous KOH. Now,
forms 1°-carbocation, while forms 2°-carbocation, which
is more stable than 1°-carbocation. Hence, is hydrolyzed more easily
than by aqueous KOH.

© 2026 GoodEd Technologies Pvt. Ltd.