s 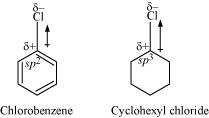
In chlorobenzene, the Cl-atom is linked to a sp hybridized carbon atom. In cyclohexyl
chloride, the Cl-atom is linked to a sp hybridized carbon atom. Now, sp hybridized carbon
has more s-character than sp hybridized carbon atom. Therefore, the former is more
electronegative than the latter. Therefore, the density of electrons of C−Cl bond near the
Cl-atom is less in chlorobenzene than in cydohexyl chloride.
Moreover, the −R effect of the benzene ring of chlorobenzene decreases the electron
density of the C−Cl bond near the Cl-atom. As a result, the polarity of the C−Cl bond in
chlorobenzene decreases. Hence, the dipole moment of chlorobenzene is lower than that
of cyclohexyl chloride.
(ii) To be miscible with water, the solute-water force of attraction must be stronger
than the solute-solute and water-water forces of attraction. Alkyl halides are polar
molecules and so held together by dipole-dipole interactions. Similarly, strong H-bonds
exist between the water molecules. The new force of attraction between the alkyl halides
and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces
of attraction. Hence, alkyl halides (though polar) are immiscible with water.
(iii) Grignard reagents are very reactive. In the presence of moisture, they react to give
alkanes.
AnTherefore, Grignard reagents should be prepared under anhydrous conditions.
10.13 Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
s 
In chlorobenzene, the Cl-atom is linked to a sp hybridized carbon atom. In cyclohexyl
chloride, the Cl-atom is linked to a sp hybridized carbon atom. Now, sp hybridized carbon
has more s-character than sp hybridized carbon atom. Therefore, the former is more
electronegative than the latter. Therefore, the density of electrons of C−Cl bond near the
Cl-atom is less in chlorobenzene than in cydohexyl chloride.
Moreover, the −R effect of the benzene ring of chlorobenzene decreases the electron
density of the C−Cl bond near the Cl-atom. As a result, the polarity of the C−Cl bond in
chlorobenzene decreases. Hence, the dipole moment of chlorobenzene is lower than that
of cyclohexyl chloride.
(ii) To be miscible with water, the solute-water force of attraction must be stronger
than the solute-solute and water-water forces of attraction. Alkyl halides are polar
molecules and so held together by dipole-dipole interactions. Similarly, strong H-bonds
exist between the water molecules. The new force of attraction between the alkyl halides
and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces
of attraction. Hence, alkyl halides (though polar) are immiscible with water.
(iii) Grignard reagents are very reactive. In the presence of moisture, they react to give
alkanes.
AnTherefore, Grignard reagents should be prepared under anhydrous conditions.