Why are aryl halides less reactive towards nucleophilic substitution
reactions than alkyl halides? How can we enhance the reactivity of aryl
halides?
reasons
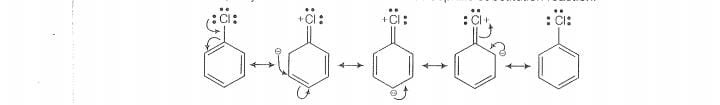
(i) In haloarenes,the lone pair of electron on halogen are in resonance with benzene ring.
S0,C —Ci bond acquires partial double bond character which strengthen C— Ci bond.
Therefore, they are less reactive towords nucleophilic substitution reaction.
(ii) In haloarenes, the carbon atom attached ta halogen is sphybridised. The sphybridised
carbon is more electronegative than sp$ hybridised carbon. This sp®-hybridised carbon
in haloarenes can hold the electron pair of C—X bond more tightly and make this
C —Cibond shorter than C — Cl bond of haloalkanes,
Since, itis difficult lo break a shorter bond than a longer bond, therefore, haloarenes are:
less reactive than haloarenes.
In haloarenes, the phenyl cation wil not be stabilised by resonance therefore S1
mechanism ruled out
(v) Because of the repulsion between the nucleophile and electron rich arenes, ary! halides
are less reactive than alkyl halides.
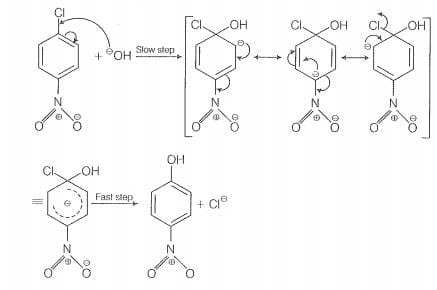
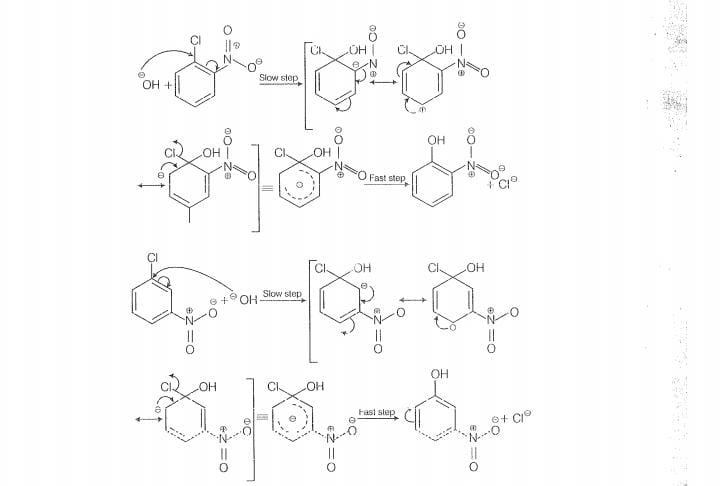
The reactivity of ary! halides can be inoreased by the presence of an electron withdrawing
group (-(NO) at ortho and para positions. However, no effect on reactivity of haloarenes
is obsorved by the presence of electron withdrawing group at meta-position, Mechanism
of the reaction is as depicted with ion.

© 2026 GoodEd Technologies Pvt. Ltd.