Some alkyl halides undergo substitution whereas some undergo
elimination reaction on treatment with bases. Discuss the structural
features of alkyl halides with the help of examples which are responsible
for this difference.
halogen atom. A transition state is formed in which carbon is bonded to two nucleophiles
and finally halogen atom is pushed out. In mechanism, substitution of nucleophile
takes place as follows
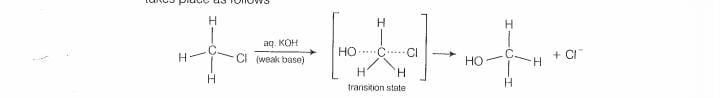
Thus, in mechanism, substitution takes place, Tertiary alkyl halides follow S
‘mechanism. in this case, tert alky! halides form 3° carbocations. Now, if the reagent used Is
‘a weak base then substitution occur while if itis @ strong base than instead of substitution
elimination ocour.
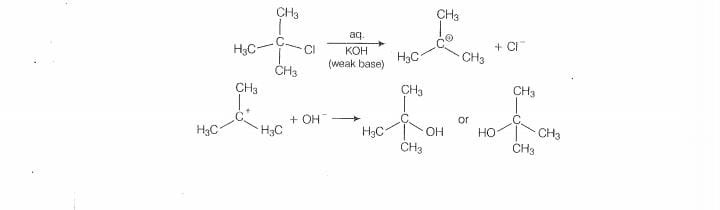
Here, the reagent used is aq. used is aq. KOH. lt is a weak base so, subsititution takes place.
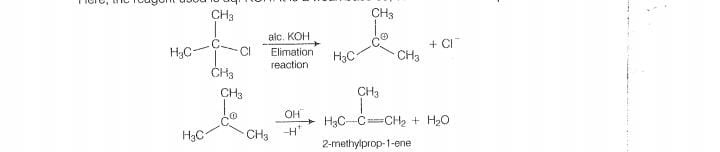
As alc. KOH is a strong bases, so elimination completes over subsitituation and alkene is formed.

© 2026 GoodEd Technologies Pvt. Ltd.