Q. 74 tert-Butylbromide reacts with aq. NaOH by S1 mechanism while
n-butylbromide reacts by S1 mechanism. Why?
tert. butyl bromide when treated with aq, NaOH, il forms tert. corbocation which is more
stable intermediate. This intermediate is further attacked by ion.
‘As tert. carbocation is highly stable so tert butylbromide follow S1 mechanism.
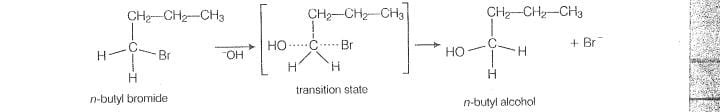
In-case of n-nutylbromide, primary carbocation is formed which is least stable so, it does not
follow S1mechanism. Here, stearic hindrance is very less so, it follow S2 mechanism. In
S2 mechanism, will atlack from backside and a transilion state is formed,
The leaving group is then pushed off the eopposite side and the product is formed.

© 2026 GoodEd Technologies Pvt. Ltd.