Which of the following compounds would undergo S1 reaction faster and why?
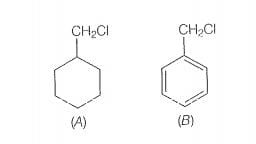
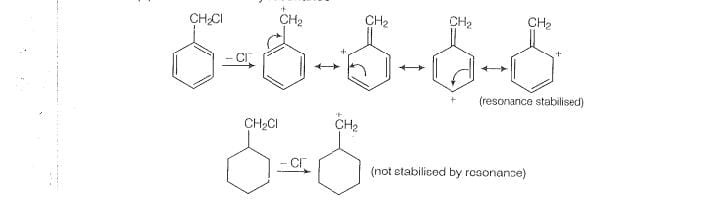
will only depend upon the stability of carbocation. Benzyl chloride on onisation gives carbocation which is resonance srabilised while the carbocation obtained from compound
(A)is not stabilised by resonance.

© 2026 GoodEd Technologies Pvt. Ltd.