Which of the following compounds will have the highest melting point
and why?
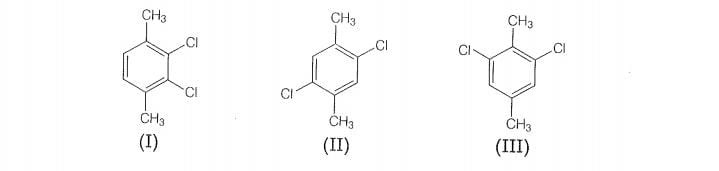
Therefore, compound (Il) is more symmetrical and it fits in the crystal lattice better than the
other two isomers and hence it has the highest mefting point than the others.

© 2026 GoodEd Technologies Pvt. Ltd.