Which of the following compounds (i) and (ii) will not react with a
mixture of NaBr and . Explain why?
(i) 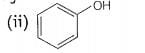
decreases the stability. Phenol will not react with a mixture of NaBr and because it is
resonance stabilised. Due to resonance, partial double bond character arises in C—O
bond of phenol and it becomes more stable than alcohol
Reaction
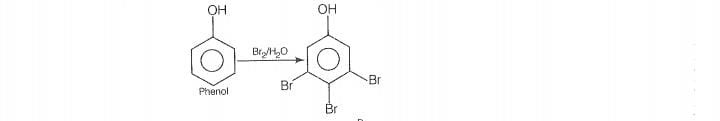

© 2026 GoodEd Technologies Pvt. Ltd.