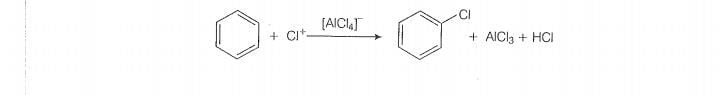
Discuss the role of Lewis acids in the preparation of aryl bromides and
chlorides in the dark.
fission in halogen molecule.
Role of Lewis acid is to produce an electrophile. The eletrophile praduce will attack on
electron rich benzene ring to produce aryl bromides and chlorides.
This electrophile wil further attack on benzene.

© 2026 GoodEd Technologies Pvt. Ltd.