Haloarenes are less reactive than haloalkanes and haloalkenes, Explain.
character. This partial double bond character of C— X bond ‘strengthen the bond. So,
haloarenes and haloalkenes are less reactive than haloalkanes.
Lets see the resormaliny structure of the haloarenes and haloalkenes
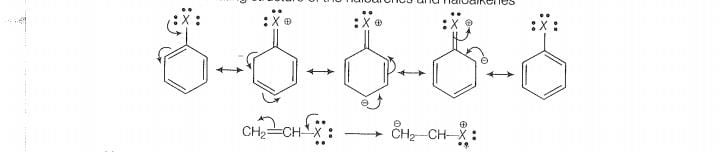
Now, more the number of resonating structure higher will be the stability of the compound
and lesser will be the reactivity. In haloarenes, more resonating structures are observed than
the haloalkenes. So, haloarenes are less reactive than haloarenes. In haloalkanes, this
— X bond is purely single bond.

© 2026 GoodEd Technologies Pvt. Ltd.