Which of the compounds will react faster in reaction with the E
ion?
this mechanism.
Sincde this carbocation is stabilised by reasonance hence it will react faster in reaction.
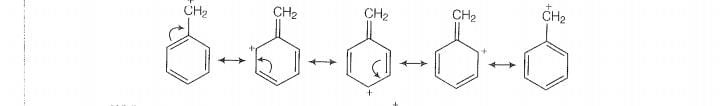
While carbocation formed in . This carbocation is compratively unstable and
not give SN1 reaction with ion.

© 2026 GoodEd Technologies Pvt. Ltd.