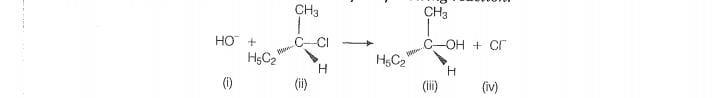
Which of the following statements are correct about the reaction
intermediate?
1. Intermediate (iii) is unstable because in this carbon is attached to 5 atoms
2. Intermediate (iii) is unstable because carbon atom is sp” hybridised
3. Intermediate (ii) is stable because carbon atom is sp’ hybridised
4. intermediate (ii) is fess stable than the reactant
For the given reaction, intermediate (li) indicates transition state, and it is highly unstable. In
this transition state, carbon atom is approximately type character as partially bonded to nucleophile and leaving group. So
it is highly unstable and compratively less stable than the reactant (i). Reactant (ii), carbon atom is sp type hence more stable than intermediate (iii).

© 2026 GoodEd Technologies Pvt. Ltd.