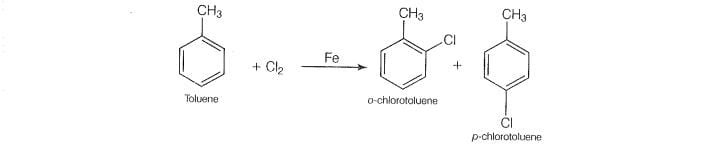
The reaction of toluene with chlorine in the presence of iron and in the absence of light produces:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. | Mixture of 2 and 3 |
o-chlorotoluene and p-chlorotoluene.

© 2026 GoodEd Technologies Pvt. Ltd.