(i) Coordination entity:
A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral molecules or negative ions ( called ligands). For example:
=cationiccomplex
=anioniccomplex
NiCO4, [Co(NH3)4Cl2]=neutralcomplex
(ii) Ligands
The neutral molecules or negatively charged ions that surround the metal atom in a coordination entity or a coordinate complex are known as ligands. For example,
NH3, H2O, OH- and Cl-. Ligands are usually polar in nature and possess at least one unshared pair of valence electrons.
(iii) Coordination number:
The total number of ligands (either neutral molecules or negative ions) that get attached to the central metal atom in the coordination sphere is called the coordination number of the central metal atom.
For example:
(a) In the complex, , there as six chloride ions attached to Pt in the coordinate sphere. Therefore, the coordination number of Pt is 6.
(b) Similarly, in the complex ,thecoordinationnumber
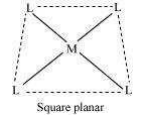
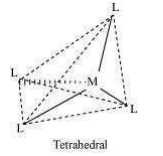
(vi) Coordination polyhedron:
Coordination polyhedrons about the central atom can be defined as the spatial arrangement of the ligands that are directly attached to the central metal ion in the coordination sphere. For example:
(a)
(b) Tetahedral
(v) Homoleptic complexes:
These are those complexes in which the metal ion is bound to only one kind of a donor group. For
eg: etc.
(vi) Heteroleptic complexes:
Heteroleptic complexes are those complexes where the central metal ion is bound to more than one type of a donor group.
For e.g. [Co(NH3)4Cl2]+ , [Cr(NH3)3(H2O)3]Cl3