Q.47 Using valence bond theory, explain the following in relation to the complexes given below
(a) Type of hybridisation
(b) lnner or outer robital comples
(c) Magnetic behaviour
(d) Spin only magnetic moment value.
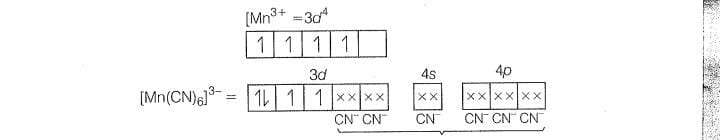
(i) hybridisation
(ii) lnner orbital complex because (n-1)d-orbitals are used.
(iii) Paramagnetic, as two unpaired electons are present.
(iv) Spin only magnetic moment ()=
(b)
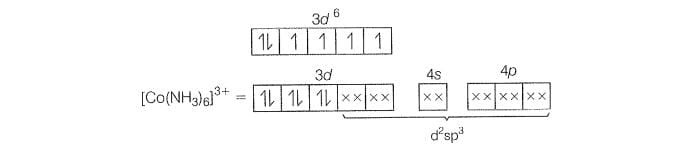
(NH pair up the unpaired 3d electons)
(i) hybridisation
(ii) lnner orbital complex because of the invovement of *n-1) d-orbital in bonding.
(iii) Diamagnetic, as no unpaired electron is preset.
(iv)
(c) 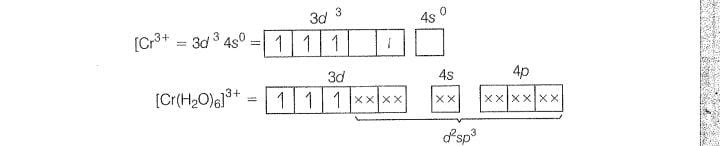
(i) hybridisation
(ii) lnner orbital complex (as(n-1) d-orbital take part)
(iii) Paramagnetic (as three unpaired electrons are present
(iv)
(d)
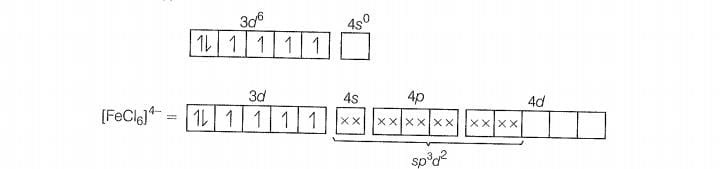
(i) hybridisation
(ii) Outer orbital complex because nd-orbitals are involved in hybridisation.
(iii) Paramagnetic (because of the presence of four unpaired electrons).
(iv)

© 2026 GoodEd Technologies Pvt. Ltd.