Match the complex species given in Column I with the possible isomerism given in Column II and assign the correct code.
|
Column I (Complex species) |
Column II (Isomerism) |
|
A. |
1. Optical isomerism |
|
B. |
2. lonisation Isomerism |
|
C. |
3. Coordination Isomerism |
|
D. |
4. Geometrical Isomerism |
Codes:
A B C D
1. 2 3 4 1
2. 3 1 5 2
3. 5 4 3 2
4. 4 1 2 3
Isomerism in coordination compounds is decided by the type of ligands, geometry of
coordination compound and arrangement of ligands.
A. shows geometrical isomerism due to the presence of two types of ligand
whose arrangement around the central metal ion.
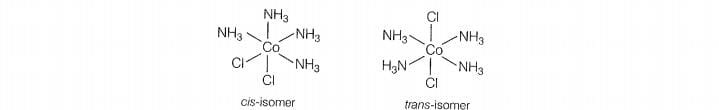
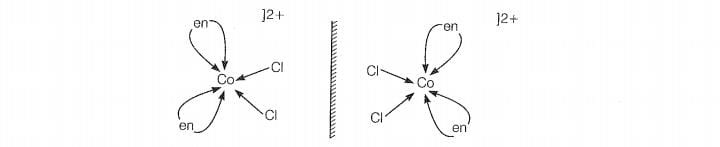
b. shows optical isomer due to its non-superimposable mirror image
relationship.
c. shows ionization isomer due to its interchanging ligand from
outside the ionization sphere.
D. shows coordination isomer due to interchanging of ligand in
between two metal ions from one coordination sphere to another coordination sphere.
Hence, the correct choice is 4.

© 2026 GoodEd Technologies Pvt. Ltd.