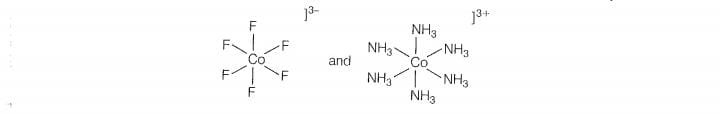
Q. 33 Why do compounds having similar geometry have different magnetic moment?
complex will show low value of magnetic moment and vice-versa, e.g. and
weak fisid ligand and NH is a strong field igand while both have similar geometry.

© 2026 GoodEd Technologies Pvt. Ltd.