Q. 28 On the basis of crystal field theory explain why Co(III) forms paramagnetic
octahedral complex with weak field ligands whereas it forms diamagnetic
octahedral complex with strong field ligands.
With weak field ligands; (pairing energy) so, the electronic configuration of Co (III)
will beti.e., it has 4 unpaired electrons and is paramagnetic.
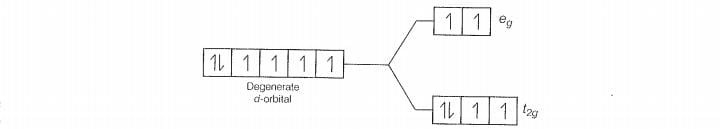
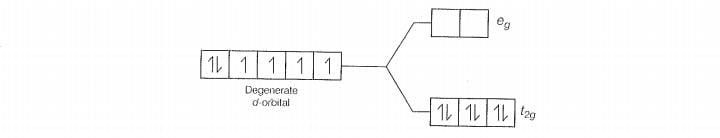
With strong field ligands (pairing energy), so pairing ocours thus, the electronic
configuration will be . It has no unpaired electrons and is diamagnetic.

© 2026 GoodEd Technologies Pvt. Ltd.