Identify the outer orbital octahedral complexes among the following that have the same number of unpaired electrons:
(Atomic numbers of Mn, Fe, Co, and Ni are 25, 26, 27, and 28 respectively)
| (a) | (b) | ||
| (c) | (d) |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Molecular orbital electronic configuration of Mn
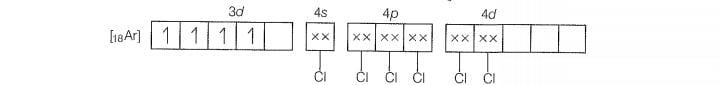
Number of unpaired electrons =4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of
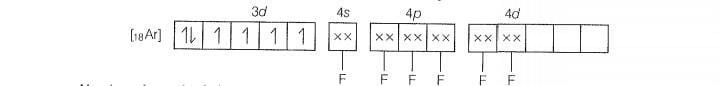
Number of unpaired electrons =4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of is
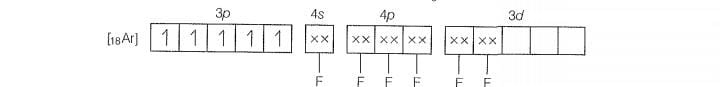
Number of unpaired electrons =4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of is
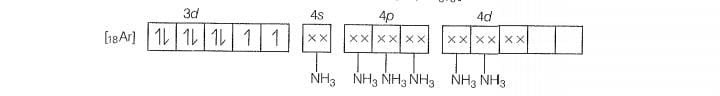
Number of unpaired electrons =4
Magnetic property = Paramagnetic
Thus, are paramagnetic having four electrons each.
Hence, correct choices are (a) (c).

© 2026 GoodEd Technologies Pvt. Ltd.