On heating lead (II) nitrate gives a brown gas “A” The gas “A” on cooling changes to colourless solid “B”. Solid “B” on heating with NO changes to a blue solid ‘C’.
Identify ‘A’, ‘B’ and ‘C and also write reactions involved and draw the structures of ‘B’ and ‘C’ .
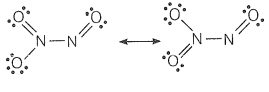
Pb(NO3)2 on heating produces a brown coloured gas which may be NO2 . Since, on reaction with N2O4 and on heating it produces N2O3 and N2O4 respectively.
Structures
(i) N2O4
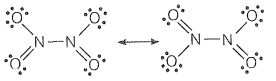
(ii) N2O3

© 2026 GoodEd Technologies Pvt. Ltd.