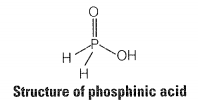
Phosphorus forms a number of oxoacids. Out of these oxoacids, phosphinic acid has strong reducing property. Write its structure and also write a reaction showing its reducing behaviour.

© 2026 GoodEd Technologies Pvt. Ltd.