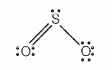
Which of the following statements are correct for SO2 gas?
| a. | It acts as a bleaching agent in moist conditions. |
| b. | Its molecule has linear geometry. |
| c. | Its dilute solution is used as a disinfectant. |
| d. | It can be prepared by the reaction of dilute H2SO4 with metal sulphide. |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)

© 2026 GoodEd Technologies Pvt. Ltd.