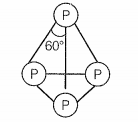
Which of the following is correct for P4 molecule of white phosphorus?
a. It has 6 lone pairs of electrons
b. It has six P — P single bonds
c. It has three P — P single bonds
d. It has four lone pairs of electrons
Choose the correct option:
1. (a, b)
2. (b, c)
3. (c, d)
4. (b, d)

© 2026 GoodEd Technologies Pvt. Ltd.