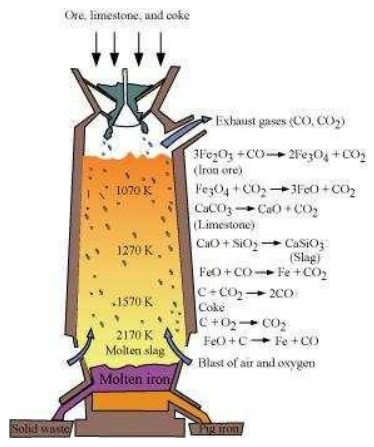
6.7 Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
The reactions taking place in the lower temperature range (500 − 800 K) in the blast furnace are:
The reactions taking place in the higher temperature range (900 − 1500 K) in the blast furnace are:
The silicate impurity of the ore is removed as slag by calcium oxide (CaO), which is formed by the decomposition of limestone ().

© 2026 GoodEd Technologies Pvt. Ltd.