5.5 What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Step 1:
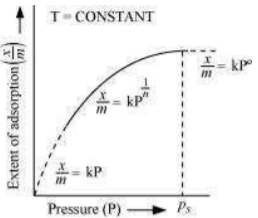
The plot between the extent of adsorption against the pressure of gas (P) at constant temperature (T) is called the adsorption isotherm.
The graph is as follows:
Step 2:
Freundlich adsorption isotherm:
Freundlich adsorption isotherm gives an empirical relationship between the quantity of gas adsorbed by the unit mass of solid adsorbent and pressure at a specific temperature.
Case I- At low pressure:
The plot is straight and sloping, indicating that the pressure directly proportional to
Case II - At high pressure:
When pressure exceeds the saturated pressure, becomes independent of P values.
Case III - At intermediate pressure:
At intermediate pressure, depends on P raised to the powers between 0 and 1. This relationship is known as the Freundlich adsorption isotherm.
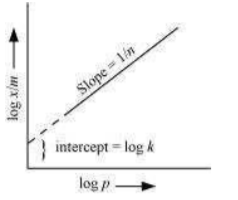
On plotting the graph between log and log P, a straight line is obtained with the slope equal to

© 2026 GoodEd Technologies Pvt. Ltd.