4.20 For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data are obtained.
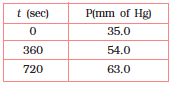
Calculate the rate constant.
The decomposition of azoisopropane to hexane and nitrogen at 543 K is represented by
the following equation.
After time, t, total pressure,
For a first order reaction,
Hence, the average value of rate constant is

© 2026 GoodEd Technologies Pvt. Ltd.