4.12 The reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table:
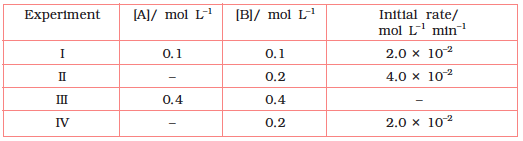
The given reaction is of the first order with respect to A and of zero order with respect to
B.
Therefore, the rate of the reaction is given by,
Rate = k [A][B]
⇒ Rate = k [A]
From experiment I, we obtain
2.0 × 10 mol L−1 min = k (0.1 mol L)
⇒ k = 0.2 min
From experiment II, we obtain
4.0 × 10 mol L min = 0.2 min[A]
⇒ [A] = 0.2 mol L−1
From experiment III, we obtain Rate
= 0.2 min × 0.4 mol L
= 0.08 mol L min
From experiment IV, we obtain
2.0 × 10 mol L−1 min = 0.2 min

© 2026 GoodEd Technologies Pvt. Ltd.