Describe how does the enthalpy of reaction remains unchanged when a catalyst is used in the reaction?
A catalyst is a substance that increases the speed of a reaction without itself undergoing any chemical change.
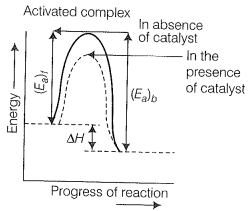
According to "intermediate complex formation theory reactants first, combined with the catalyst to form an intermediate complex that is short-lived and decomposes to form the products and regenerating the catalyst.
The intermediate formed has much lower potential energy than the intermediate complex formed between the reactants in the absence of the catalyst.
Thus, the presence of a catalyst lowers the potential energy barrier and the reaction follows a new alternate pathway which requires less activation energy.
We know that the lower the activation energy, the faster is the reaction because more reactant molecules can cross the energy barrier and change into products.
Enthalpy, H is a state function. Enthalpy of reaction, i.e., the difference in energy between reactants and product is constant, which is clear from the potential energy diagram.
The potential energy diagram of catalyzed reaction is given as

© 2026 GoodEd Technologies Pvt. Ltd.