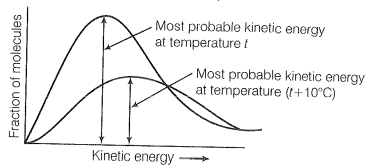
What happens to most probable kinetic energy and the energy of activation with an increase in temperature?
Kinetic energy is directly proportional to the absolute temperature and the number of molecules possessing higher energies increases with an increase in temperature, i.e., most probable kinetic energy increases with an increase in temperature. The energy of activation is related to temperature by the following Arrhenius equation
Thus, it also shows an increase with rising in temperature

© 2026 GoodEd Technologies Pvt. Ltd.