The correct statements among following about Maxwell, Boltzmann distribution of energy is -
| (a) | The fraction of molecules with the most probable kinetic energy decreases at higher temperatures |
| (b) | The fraction of molecules with the most probable kinetic energy increases at higher temperatures |
| (c) | Most probable kinetic energy increases at higher temperatures |
| (d) | Most probable kinetic energy decreases at higher temperatures |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Hint: Increase in temperature, the peak shifts forward but downward.
Distribution of kinetic energy may be described by plotting a graph of the fraction of molecules versus kinetic energy.
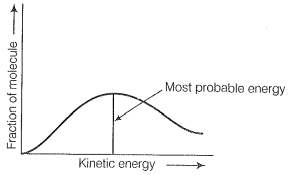
The kinetic energy of the maximum fraction of a molecule is known as the most probable kinetic energy. It is important to note that with an increase in temperature, the peak shifts forward but downward.
This means that with an increase in temperature,
(i) Most probable kinetic energy increases.
(ii) The fractions of molecules possessing the most probable kinetic energy decreases.

© 2026 GoodEd Technologies Pvt. Ltd.