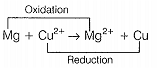
For the cell, Mg | Mg2+ || Cu2+ | Cu
a. Mg acts as cathode
b. Cu acts as cathode
c. The cell reaction is \(Mg + Cu^{2+} \rightarrow Mg^{2+} + Cu\)
d. Cu is the oxidising agent
The correct choice among the given is:
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, d) |

© 2026 GoodEd Technologies Pvt. Ltd.