3.7 Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Conductivity of a solution is defined as the conductance of a solution of 1 cm in length and area of cross-section 1 sq. cm. The inverse of resistivity is called conductivity or specific conductance. It is represented by the symbol κ. If ρ is resistivity, then we can write:
Step 2:
The conductivity of a solution at any given concentration is the conductance (G) of one unit volume of solution kept between two platinum electrodes with the unit area of cross-section and at a distance of unit length.
i.e.,
(Since a=1, l=1)
Step 3:
Conductivity always decreases with a decrease in concentration, both for weak and strong electrolytes. This is because the number of ions per unit volume that carry the current in a solution decreases with a decrease in concentration.
Molar conductivity:
Molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.
Now, l = 1 and A = V (volume containing 1 mole of the electrolyte).
Step 4:
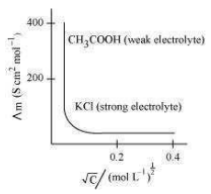
Molar conductivity increases with a decrease in concentration. This is because the total volume V of the solution containing one mole of the electrolyte increases on dilution. The variation of for strong and weak electrolytes is shown in the following plot:

© 2026 GoodEd Technologies Pvt. Ltd.