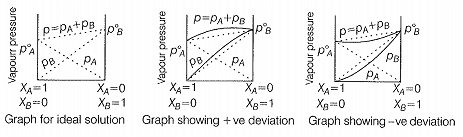
The solutions which obey Raoult's law over the entire range of concentration are known as ideal solutions. For an ideal solution = O and = O. The ideal behaviour of the solutions can be explained by considering two components A and B.
In pure components, the intermolecularattractive interactionswill be of A—A type and B—B type, whereas in the binary solutions in addition to these two, A—B type ofinteraction will also be present. If A—A and B—B intermolecular forces are nearly equal to those between A—B, this leads to the formation of ideal solution e.g., solution of n-hexane and n-heptane.
When a solution does not obey-Raoult’s law over the entire range of concentration, then it is called non-ideal solution. The vapour pressure of such a solution is either higher or lower, than that predicted by Raoult’s law.
If it is higher, the solution exhibits positive deviation and if it is lower it exhibits negative deviation from Raoult’s law. In case of positive deviation, A—B interactions are weaker than those between A—A or B--B. i.e., the attractive forces betweensolute solvent molecules are weaker than those between solute-solute and solvent-solvent molecules e.g., mixture of ethanol and acetone.
For such solutions = + Ve and = + Ve mixing
On the other hand, in case of negative deviationthe intermolecular attractive forces between A—A and B—B are weaker than those between A—B molecules. Thus, the escaping tendency of A and B types of molecules from the solution becomesless than from the pure liquids i.e., mixture of chloroform and acetone.
For such solution = - ve and = - ve