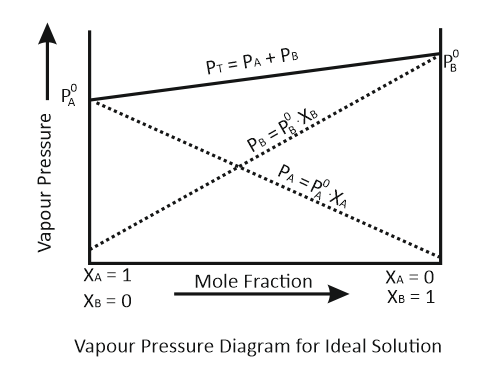
For a binary ideal liquid solution, the curve that represents the variation in total vapor pressure versus composition of the solution is:
| a. |  |
b. |  |
| c. |  |
d. |  |
The correct option is:
1. a and b
2. b and c
3. c and d
4. a and d

© 2026 GoodEd Technologies Pvt. Ltd.