Select the correct option based on statements below:
| Assertion (A): | Total number of octahedral voids present in the unit cell of cubic close packing including the one that is present at the body center, is four. |
| Reason (R): | Besides the body center there is one octahedral void present at the center of each of the six faces of the unit cell and each of which is shared between two adjacent unit cells. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Cubic close packing means FCC. In FCC, atoms are present at face centre and corner of each unit cell which creates octahedral void at each body centre ard all twelve edges of a unit cell as shown below:
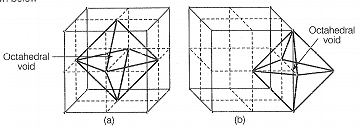
In FCC, 4 atoms are present, hence, four octahedral voids are present.
Thus, Assertion is a correct statement but Reason is an incorrect statement.

© 2026 GoodEd Technologies Pvt. Ltd.