Match the items given in Column I with the items given in Column II.
| Column I | Column II |
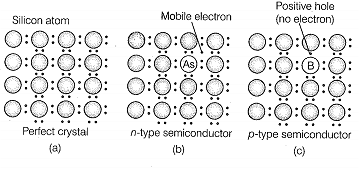
| A. Mg in solid state | 1. p-type semiconductor |
| B. MgCl2 in molten state | 2. n-type semiconductor |
| C. Silicon with phosphorus | 3. Electrolytic conductors |
| D. Germanium with boron | 4. Electronic conductors |
Codes
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 2 | 1 |

© 2026 GoodEd Technologies Pvt. Ltd.