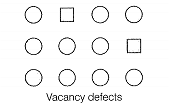
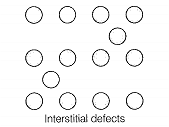
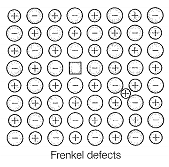
Match the defects given in Column I with the statements given in Column II.
| Column I | Column II |
| A. Simple vacancy defect | 1. Shown by non-ionic solids and increases the density of the solid |
| B. Simple interstitial defect | 2. Shown by ionic solids and decreases the density of the solid |
| C. Frenkel defect | 3. Shown by non-ionic solids and decreases the density of the solid |
| D. Schottky defect | 4. Shown by ionic solids and density of the solid remains the same |
Codes
| A | B | C | D | |
| 1. | 3 | 1 | 4 | 2 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 3 | 2 |


© 2026 GoodEd Technologies Pvt. Ltd.