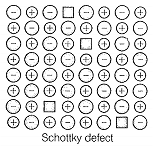
Schottky defect is observed in crystals when:
| 1. | Some cations move from their lattice site to interstitial sites. |
| 2. | Equal number of cations and anions are missing from the lattice. |
| 3. | Some lattice sites are occupied by electrons. |
| 4. | Some impurities are present in the lattice. |

© 2026 GoodEd Technologies Pvt. Ltd.