In the presence of peroxide addition of HBr to propene takes place according to anti Markownikoff's rule but peroxide effect is not seen in the case of HCl and HI. Explain.
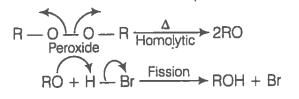
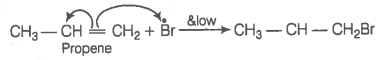
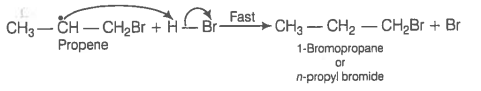

© 2026 GoodEd Technologies Pvt. Ltd.