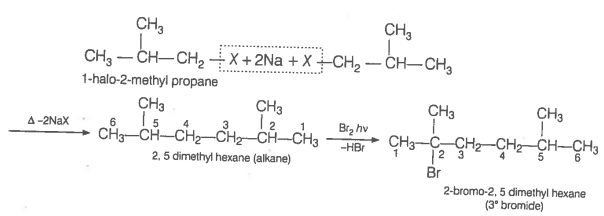
An alkane is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination this alkane yields a single isomer of a tertiary bromide. Write the structure of alkane and the tertiary bromide.
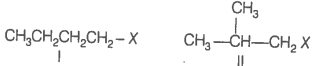

© 2026 GoodEd Technologies Pvt. Ltd.