Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Classify the following species as electrophile and nucleophiles.
1.
2. 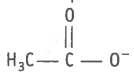
3.
4.
5.
6.
7.
8.
(i) , (ii) 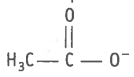 , (vi) , (vii) , (viii)
, (vi) , (vii) , (viii)

© 2026 GoodEd Technologies Pvt. Ltd.