The intermediate carbocation formed in the reactions of HI, HBr and HCl with propene is the same and the bond energy of HCl, HBr and HI is respectively. What willl be the order of reactivity of these halogen acids?
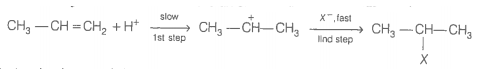

© 2026 GoodEd Technologies Pvt. Ltd.