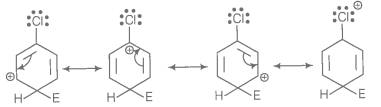
For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring ______
1. deactivates the ring by inductive effect
2. deactivates the ring by resonance
3. increases the charge density at ortho and para position relative to meta position by resonance.
4. directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.

© 2026 GoodEd Technologies Pvt. Ltd.