Question 12.17:
Explain the terms Inductive and Electromeric effects. Which electron displacement effect
explains the following correct orders of acidity of the carboxylic acids?
(a)ClCCOOH > ClCHCOOH > ClCHCOOH
(b)CHCHCOOH > (CH3)CHCOOH > (CH)C.COOH
Inductive effect
The permanent displacement of sigma (σ) electrons along a saturated chain, whenever an
electron withdrawing or electron donating group is present, is called inductive effect.
Inductive effect could be + I effect or – I effect. When an atom or group attracts electrons
towards itself more strongly than hydrogen, it is said to possess – I effect.
For example,
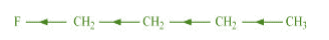
When an atom or group attracts electrons towards itself less strongly than hydrogen, it is
said to possess + I effect.
For example,
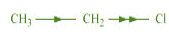
Electrometric effect
It involves the complete transfer of the shared pair of π electrons to either of the two
atoms linked by multiple bonds in the presence of an attacking agent. For example,
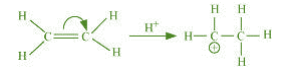
Electrometric effect could be + E effect or – E effect.
+ E effect: When the electrons are transferred towards the attacking reagent
– E effect: When the electrons are transferred away from the attacking reagent
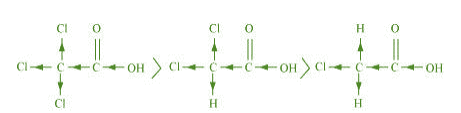
(a) ClCCOOH > ClCHCOOH > ClCHCOOH
The order of acidity can be explained on the basis of Inductive effect (– I effect). As the
number of chlorine atoms increases, the – I effect increases. With the increase in – I
effect, the acid strength also increases accordingly.
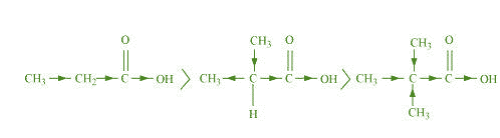
(b) CHCHCOOH > (CH) CHCOOH > (CH) C.COOH
The order of acidity can be explained on the basis of inductive effect (+ I effect). As the
number of alkyl groups increases, the + I effect also increases. With the increase in + I
effect, the acid strength also increases accordingly.

© 2026 GoodEd Technologies Pvt. Ltd.