Question 12.16:
For the following bond cleavages, use curved-arrows to show the electron flow and classify
each as homolysis or heterolysis. Identify reactive intermediate produced as free radical,
carbocation and carbanion.
(a) 
(b) 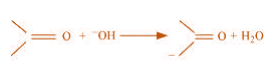
(c) 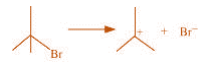
(d) 
(a) The bond cleavage using curved-arrows to show the electron flow of the given
reaction can be represented as


(b) It is an example of homolytic cleavage as one of the shared pair in a covalent bond goes
with the bonded atom. The reaction intermediate formed is a free radical.
(b) The bond cleavage using curved-arrows to show the electron flow of the given
reaction can be represented as
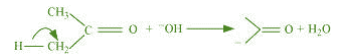
(b) The bond cleavage using curved-arrows to show the electron flow of the given
reaction can be represented as
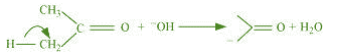
It is an example of heterolytic cleavage as the bond breaks in such a manner that the
shared pair of electrons remains with the carbon of propanone. The reaction intermediate
formed is carbanion.
(c) The bond cleavage using curved-arrows to show the electron flow of the given
reaction can be represented as
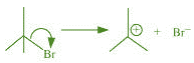
It is an example of heterolytic cleavage as the bond breaks in such a manner that the
shared pair of electrons remains with the bromine ion. The reaction intermediate formed
is a carbocation.
(d) The bond cleavage using curved-arrows to show the electron flow of the given
reaction can be represented as

It is a heterolytic cleavage as the bond breaks in such a manner that the shared pair of
electrons remains with one of the fragments. The intermediate formed is a carbocation.

© 2026 GoodEd Technologies Pvt. Ltd.