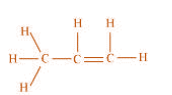When an alkyl group is attached to a π system, it acts as an electron-donor group by the
process of hyperconjugation. To understand this concept better, let us take the example
of propene. .
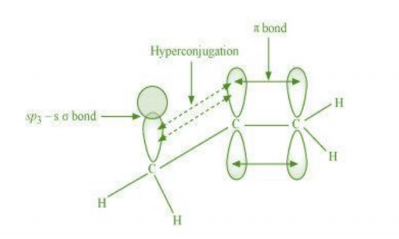
ln hyperconjugation, the sigma electrons of the C-H bond of an alkyl group of an alkyl group are delocalised.
This group is directly attached to an atom, of an unsaturated system. The delocalisation occurs because of a
artial overlap of a sp-s sigma bond orbital with an empty p orbital of the n bond of an adjacent carbon atom.
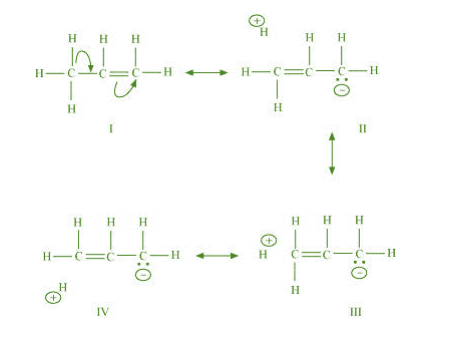
The process of hyperconjugation in propene is shown as follows:
This type of overlap leads to a delocalisation (also known as no-bond resonance) of the π
electrons, making the molecule more stable.