Indicate the σ and π bonds in this molecule
1. =4, = 2, = 1, and = 1
2. =2, = 4, = 1, and = 1
3. =1, = 4, = 2, and = 1
4. =1, = 4, = 1, and i = 2
Explanation:
Step 1: Below is the structure of
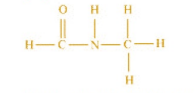

© 2026 GoodEd Technologies Pvt. Ltd.